Example: Detecting Parkinson using Smart Phone Sensors
This notebook evaluates FLASC to reproduce a finding by Mapper on a dataset containing smart phone sensor measurements from healthy individuals and Parkinson’s patients.
The data was part of a Kaggle challenge issued by the Michael J. Fox foundation. It contains samples recorded roughly between December 2011 and March 2012 for 9 parkinson’s patients and 7 healthy controls roughly matched for age and gender.
The subjects were asked to carry a supplied Android smartphone on their person for at least one charge-cycle per day (about 4-6 hours) during 8 weeks, as consistently as possible. The data was recorded at most once per second in various forms:
Audio
Accelerometry
Compass
Ambient light
Proximity
Battery level
GPS
In this notebook, we try to re-construct a Mapper network on this data presented by Anthony Bak from (then called) Ayasdi at a Stanford Seminar:
Loading Data
[1]:
%load_ext autoreload
%autoreload 2
[2]:
import pandas as pd
import numpy as np
import seaborn as sns
import matplotlib.pyplot as plt
from matplotlib import collections as mc
from flasc import FLASC
from sklearn.metrics import pairwise_distances
from sklearn.preprocessing import StandardScaler
from lib.plotting import *
%matplotlib inline
palette = configure_matplotlib()
Other than the file-name, I cannot find details on how the dataset used is computed from the raw-data provided in the Kaggle competition. The first column identifies samples from the same participant and the second column indicates whether that participant is a control or a patient. The other columns contain unspecified real and imaginary Fourier transform coefficients.
[3]:
df = pd.read_csv("./data/parkinson/all_FFT_3_hr_conv_60_s_128_harmonics.tsv", sep="\t")
df.head()
[3]:
| Sample | Disease | Re_1 | Re_2 | Re_3 | Re_4 | Re_5 | Re_6 | Re_7 | Re_8 | ... | Im_119 | Im_120 | Im_121 | Im_122 | Im_123 | Im_124 | Im_125 | Im_126 | Im_127 | Im_128 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | Apple | control | 2.27452 | -2.98812 | -3.95080 | -2.98221 | -3.57399 | -3.50384 | -3.47639 | -5.30028 | ... | 1.46128 | 1.80453 | 1.54919 | 1.70423 | 1.47270 | 1.44480 | 1.78379 | 1.49810 | 1.74807 | 1.69820 |
| 1 | Apple | control | 2.27566 | -3.22163 | -3.73390 | -3.32565 | -3.31341 | -3.44289 | -3.48040 | -4.57056 | ... | -1.48798 | -1.48112 | -1.38925 | -2.06651 | -1.24703 | -1.45957 | -1.64805 | -1.65060 | -1.27808 | -1.48379 |
| 2 | Apple | control | 2.27763 | -2.75829 | -2.89338 | -4.92310 | -3.98356 | -3.26541 | -3.99159 | -3.39677 | ... | 1.65722 | 1.58372 | 1.74900 | 1.45614 | 1.60677 | 1.63392 | 1.56392 | 1.64865 | 1.66224 | 1.61040 |
| 3 | Apple | control | 2.28332 | -2.25339 | -2.65027 | -4.88183 | -3.48719 | -3.38162 | -3.85605 | -4.81275 | ... | 1.62460 | 1.57034 | 1.31235 | 1.67700 | 1.50308 | 1.55768 | 1.53018 | 1.49809 | 1.62076 | 1.50912 |
| 4 | Apple | control | 2.29163 | -2.10240 | -3.99083 | -3.49268 | -3.49333 | -3.78279 | -3.46723 | -3.34228 | ... | 1.70419 | 1.67215 | 1.60885 | 1.47521 | 1.52391 | 1.57575 | 1.63493 | 1.61496 | 1.49248 | 1.57355 |
5 rows × 258 columns
Reproducing the Mapper network
The first goal is to reproduce the Y-shaped Mapper network. In the presentation, they report using a variance normalized Euclidean distance and \(l_{infinity}\) centrality lens.
[4]:
X = StandardScaler(with_mean=False).fit_transform(df.iloc[:, 2:])
distances = pairwise_distances(X, metric='euclidean')
eccentricity = distances.max(axis=1)
diseased = df.Disease != 'control'
Computing a covering
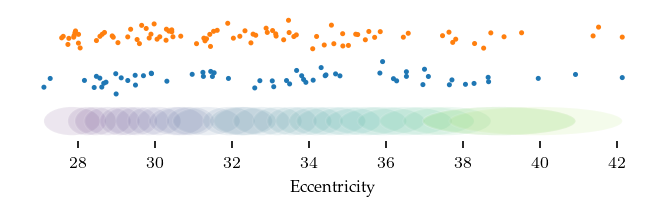
Based on the presentation, their covering was equalized and parameterized with a resolution of \(30\) segments, a \(4.0\times\) gain. I have interpreted that gain value to mean an overlap of \(3/4\) segment.
The image below shows the resulting covering, with samples from diseased participants in orange and control samples in blue.
[5]:
from gtda.mapper import OneDimensionalCover
from matplotlib.patches import Ellipse
resolution = 30
overlap = 3/4
y = diseased + np.ones(len(eccentricity)) + np.random.normal(0, 0.14, len(eccentricity))
def plot_cover(lens, starts, ends):
sized_fig(1, aspect=0.2)
cmap = plt.get_cmap('viridis')
norm = plt.Normalize(lens.min(), lens.max())
plt.scatter(
lens,
y,
2, diseased, cmap='tab10', vmax=10)
for i, (begin, end) in enumerate(zip(starts, ends)):
if i == 0:
begin = lens.min()
if i == len(starts) - 1:
end = lens.max()
plt.gca().add_patch(r)
r = Ellipse(
((begin+end)/2, 0),
end-begin,
0.66,
alpha=0.1,
facecolor=cmap(norm((begin+end)/2))
)
plt.gca().add_patch(r)
plt.yticks([])
plt.xlabel('Eccentricity')
cover = OneDimensionalCover(
kind='balanced', n_intervals=resolution, overlap_frac=overlap
).fit(eccentricity)
starts, ends = cover.left_limits_, cover.right_limits_
plot_cover(eccentricity, starts, ends)
Clustering each segment
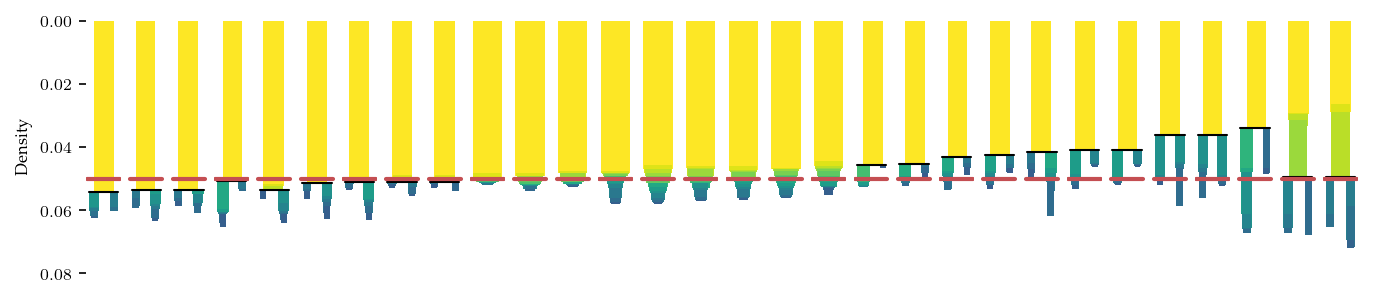
We use HDBSCAN* with a minimum cluster size of 5 samples for Mappers clustering step. The precise clustering is likely different from the first-histogram-gap approach used for the original Mapper network.
The condensed hierarchies are shown below, and indicate an X-shaped structure. To achieve the Y-shaped network, we applied a cluster_selection_epsilon of \(1/0.05\), which merges the most central branches. In our experience it is essential to visualize these kind of hierarchies when constructing Mapper networks. Without them it is very difficult to reason about which clusters are detected and how they relate to each other.
[6]:
from hdbscan import HDBSCAN
def segment_tree(dists):
c = HDBSCAN(
metric='precomputed',
min_cluster_size=5,
allow_single_cluster=True,
cluster_selection_method='leaf',
cluster_selection_epsilon=1/0.05,
).fit(dists)
labels = c.labels_.copy()
# ALl points are noise -> one big cluster
if np.all(labels == -1):
return np.zeros(dists.shape[0]), c
return labels, c
pullback_sets = [
np.where((eccentricity >= begin) & (eccentricity <= end))[0]
for begin, end in zip(starts, ends)
]
distances_sets = [
distances[:, pts][pts, :]
for pts in pullback_sets
]
label_sets, c_sets = zip(*[
segment_tree(dist)
for dist in distances_sets
])
sized_fig(2, aspect=0.2)
n_sets = len(pullback_sets)
for i in range(n_sets):
plt.subplot(1, n_sets, i+1)
c = c_sets[i]
c.condensed_tree_.plot(colorbar=False)
plt.ylim([0.08, 0])
if i > 0:
plt.axis('off')
else:
plt.ylabel('Density')
plt.plot(plt.xlim(), [0.05, 0.05], 'r-', linewidth=2)
plt.show()
Converting to Network structure
The clusters from the previous step are converted into a NetworkX graph which we use for plotting.
[7]:
import networkx as nx
from matplotlib import collections as mc
cnt = 0
def nodes_and_edges(pullback_sets, label_sets, starts, ends):
global cnt
cnt = 0
def inc():
global cnt
cnt += 1
return cnt
node_attrs = {
inc(): {
'level': int(i),
'start': float(start),
'end': float(end),
'diseased': float(diseased[pts[label==j]].mean()),
'eccentricity': float(eccentricity[pts[label==j]].mean()),
'size': int(np.sum(label == j)),
'points': pts[label==j].astype(int).tolist()
}
for i, (pts, label, start, end) in enumerate(zip(
pullback_sets, label_sets,
np.concatenate((starts, starts)),
np.concatenate((ends, ends))
))
for j in np.unique(label) if j >= 0
}
nodes = np.arange(1, len(node_attrs), dtype=int).tolist()
edges = []
edge_attrs = {}
for i in node_attrs.keys():
for j in node_attrs.keys():
if i == j:
continue
overlap = len(np.intersect1d(node_attrs[i]['points'], node_attrs[j]['points']))
if overlap > 0:
edges += [(i, j)]
edge_attrs[(i,j)] = {'weight': float(overlap)}
return nodes, node_attrs, edges, edge_attrs
def to_nx_graph(nodes, node_attrs, edges, edge_attrs):
g = nx.Graph()
g.add_nodes_from(nodes)
g.add_edges_from(edges)
nx.set_node_attributes(g, node_attrs)
nx.set_edge_attributes(g, edge_attrs)
return g
def plot_network_by(attr, **kwargs):
color = [*nx.get_node_attributes(g, attr).values()]
plt.gca().add_collection(
mc.LineCollection(
list(zip(zip(xs[source], ys[source]), zip(xs[target], ys[target]))),
linewidths=.5, color='k', zorder=0, alpha=0.2
)
)
plt.scatter(xs, ys, size, color, cmap='turbo', **kwargs)
plt.title(attr)
plt.axis('off')
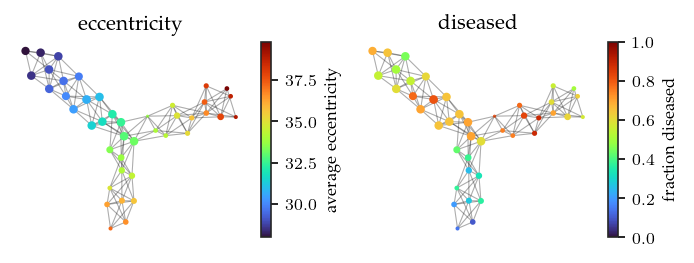
The resulting Mapper network does indeed consist of three branches. One more central branch and two branches with higher eccentricity. While the colors are reversed, we also find one branch with more controls and two branches with more patients. All in all, this reproduced mapper network appears quite similar to the original one.
[8]:
nodes, node_attrs, edges, edge_attrs = nodes_and_edges(
pullback_sets, label_sets, starts, ends
)
g = to_nx_graph(nodes, node_attrs, edges, edge_attrs)
pos = nx.nx_agraph.graphviz_layout(g, prog="neato")
for n, p in pos.items():
g.nodes[n]['pos'] = p
xs, ys = (np.asarray(x) for x in zip(*pos.values()))
source, target = [np.asarray(x) - 1 for x in zip(*edges)]
size = [*nx.get_node_attributes(g, 'size').values()]
norm = plt.Normalize(np.min(size), np.max(size))
size = [norm(s) * 10 + 0.2 for s in size]
sized_fig(1, 0.618/2)
plt.subplot(1, 2, 1)
plot_network_by('eccentricity')
plt.colorbar(label='average eccentricity')
plt.subplot(1, 2, 2)
plot_network_by('diseased', vmin=0, vmax=1)
plt.colorbar(label='fraction diseased')
plt.show()
FLASC
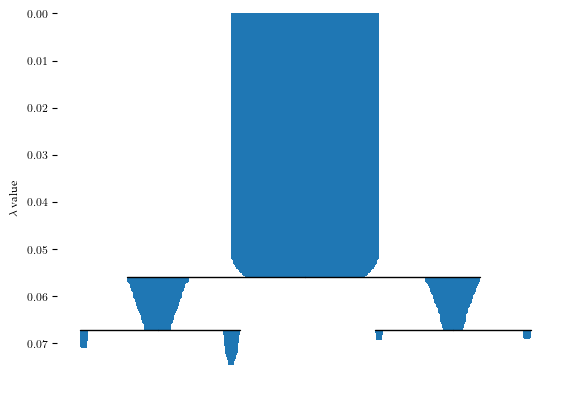
Now, lets analyze this data with FLASC. Firstly, for detecting clusters, we set min samples=2 with min cluster size=5 and enable allow single cluster because many points join the condensed hierarchy at the root segment, indicating that the density maxima do not describe a large part of the dataset. Secondly, for detecting branches, we use min branch size=5 using the core detection method.
[9]:
b = FLASC(
min_samples=2,
min_cluster_size=5,
allow_single_cluster=True,
label_sides_as_branches=True
).fit(X)
g2 = b.cluster_approximation_graph_
[10]:
b.condensed_tree_.plot()
plt.show()
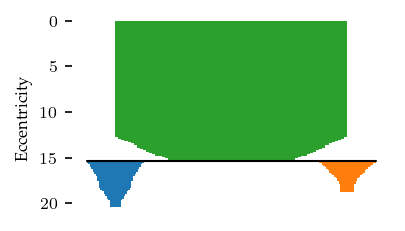
These settings result in one cluster and two branches, as shown in the branch condensed hierarchy:
[11]:
sized_fig(1/2)
n_clusters = len(b.cluster_persistence_)
for i in range(n_clusters):
plt.subplot(1, n_clusters, i+1)
b.branch_condensed_trees_[i].plot()
if i ==0:
plt.ylabel('Eccentricity')
else:
plt.axis('off')
plt.show()
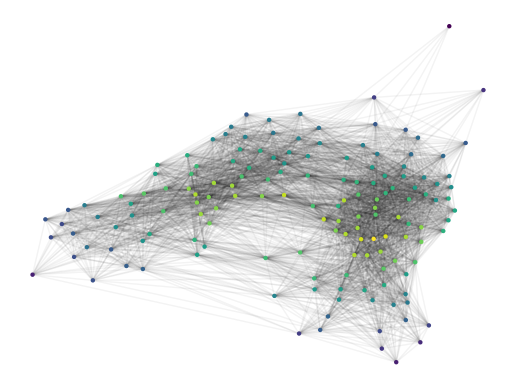
Visualizing the cluster approximation graph coloured by the centrality reveals a hamburger-bun structure. There appear to be two distinct regions with centrality maxima, which would match the X-shaped mapper network we saw before.
[14]:
g2.plot(
node_size=10,
node_color='centrality',
edge_alpha=0.05,
)
plt.show()
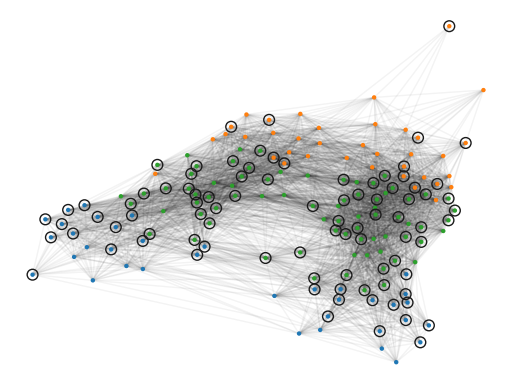
In the figure below, the data points are coloured by their FLASC label. The surrounding circles indicate which observations are from a diseased participant. Visually, most controls (points without surrounding circles) lie in the blue branch. These points form the controls-branch in the Mapper network. The green subgroup indicates the most-central branch in the Mapper network. The orange branch forms the remaining Mapper branch.
[15]:
g2.plot(
node_size=10,
edge_alpha=0.05,
)
plt.scatter(
g2._xs[g2.point_mask & diseased],
g2._ys[g2.point_mask & diseased],
60,
edgecolors='k',
facecolors='none',
marker='o',
)
plt.show()
Comparing to the Mapper network
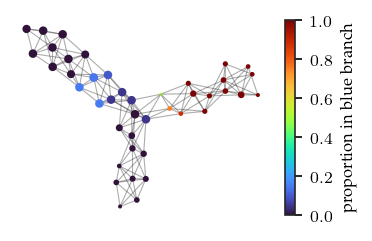
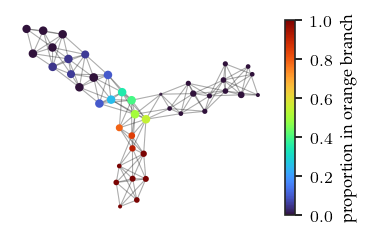
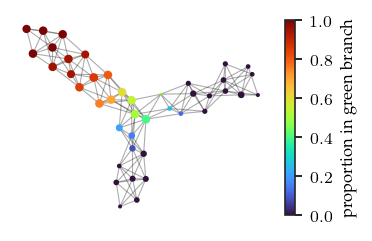
We can color the mapper network by the datapoint’s FLASC labels. Specifically, we color by the proportion of each node’s points that belongs to each FLASC subgroup. These figures confirm the observations made previously.
[16]:
memberships = np.zeros((X.shape[0], len(np.unique(b.labels_))))
for pts in b.cluster_points_:
label_values = np.unique(b.labels_[pts])
for l in label_values:
mask = b.labels_[pts] == l
memberships[pts[mask], l] = 1
[17]:
branches = ['blue','orange','green']
for i, branch in enumerate(branches):
sized_fig()
branch_score = [
memberships[pts[label==j], i].mean()
for pts, label in zip(
pullback_sets, label_sets,
)
for j in np.unique(label) if j >= 0
]
plt.gca().add_collection(
mc.LineCollection(
list(zip(zip(xs[source], ys[source]), zip(xs[target], ys[target]))),
linewidths=.5, color='k', zorder=0, alpha=0.2
)
)
plt.scatter(xs, ys, size, branch_score, cmap='turbo', vmin=0)
plt.colorbar(label=f'proportion in {branch} branch')
plt.axis('off')
plt.show()